Abstract
Introduction: Reactivation of hepatitis B virus (HBV) is an identified risk associated with immunochemotherapy for non-Hodgkin lymphoma (NHL) in patients (pts) with resolved HBV infection. This study aimed to evaluate the risk of HBV reactivation and explore risk factors in NHL pts with resolved HBV infection who received anti-CD20 antibody (obinutuzumab or rituximab)-containing immunochemotherapy in the phase III GOYA and GALLIUM studies (involving pts with previously untreated diffuse large B-cell lymphoma [DLBCL] and indolent NHL, respectively).
Methods: Pts were randomized to receive induction with either obinutuzumab (1000 mg on days 1, 8, and 15 of cycle 1, and day 1 of cycles 2‒8) or rituximab (375 mg/m2 on day 1 of each cycle) in combination with CHOP (GOYA and GALLIUM), CVP (GALLIUM), or bendamustine (GALLIUM) for 6-8 cycles. In GALLIUM, induction was followed by obinutuzumab or rituximab maintenance. Baseline screening was performed locally for hepatitis B surface antigen (HBsAg) and antibody against hepatitis B core antigen (anti-HBc). Pts positive for HBsAg were excluded, while those with resolved HBV infection (HBsAg-negative but anti-HBc-positive) could be enrolled if they had baseline HBV DNA <29 IU/mL. To prevent HBV reactivation leading to hepatitis, HBV DNA monitoring was performed every 21 days until the end of induction and monthly during maintenance/follow-up to 1 year after the last dose. In case of HBV reactivation (defined as confirmed quantifiable HBV DNA ≥29 IU/mL, as assessed by real-time quantitative PCR in a central laboratory), immunochemotherapy was withheld and anti-HBV nucleos(t)ide analog treatment (NAT; including entecavir, lamivudine, tenofovir, or adefovir) was started (preemptive NAT). Immunochemotherapy was restarted if HBV DNA decreased to undetectable levels (<10 IU/mL) or if reactivation was not confirmed by HBV DNA retesting. If HBV DNA exceeded 100 IU/mL while a pt was receiving NAT, immunochemotherapy was discontinued. Antiviral prophylaxis (NAT), started before any HBV reactivation, was also allowed based on the discretion of the participating physician. Pts not confirmed by HBV DNA retesting before starting NAT were not classified as having HBV reactivation. Cox proportional hazards models were used to identify subsets of independent factors related to the risk of HBV reactivation. Factors with p<0.2 by univariate analysis, age, and gender were included in the multivariate analysis.
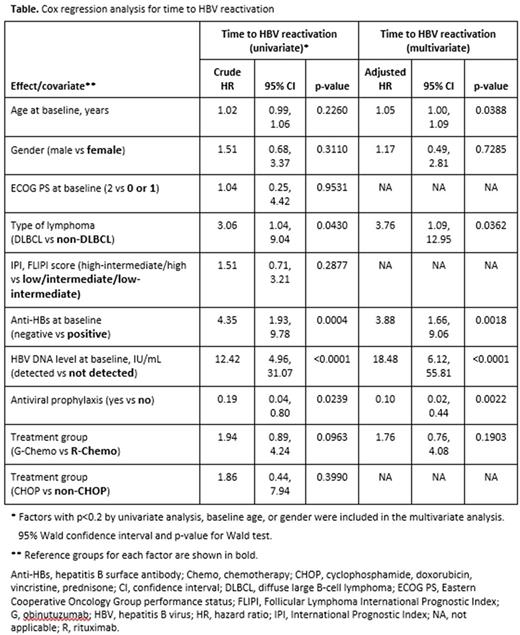
Results: Of 2797 evaluable pts (GOYA, 1407; GALLIUM, 1390), 326 pts were seropositive for anti-HBc (207 were seropositive for antibodies against HBsAg) and 11 had detectable but non-quantifiable HBV DNA (10 to <29 IU/mL) at baseline. At a median follow-up of 546.5 days (IQR, 282-817), 27 pts had HBV reactivation (21 had HBV DNA ≥100 IU/mL), occurring at a median time of 125 days (IQR, 85-331) after first dose. Among the 233 pts who did not receive antiviral prophylaxis, 25 had HBV reactivation with a median HBV DNA peak of 342 IU/mL (IQR, 101-1230); all 25 pts received preemptive NAT. Among the 93 pts who received antiviral prophylaxis (56 before starting immunochemotherapy and 37 thereafter), 2 had HBV reactivation: 1 after stopping NAT and 1 who was still on NAT (lamivudine). None of the 27 reactivated pts developed HBV-related hepatitis. Multivariate analysis (Table) showed that baseline age and type of lymphoma (DLBCL) were independent risk factors for HBV reactivation (adjusted HR [95% CI]: 1.05 [1.00, 1.09] and 3.76 [1.09, 12.95], respectively). Seronegativity for anti-HBs and detectable but non-quantifiable HBV DNA at baseline were also independent risk factors for reactivation (adjusted HR [95% CI]: 3.88 [1.66, 9.06] and 18.48 [6.12, 55.81], respectively). Antiviral prophylaxis was associated with a significantly reduced risk of HBV reactivation (adjusted HR [95% CI]: 0.10 [0.02, 0.44]).
Conclusions: HBV DNA monitoring-guided preemptive NAT was effective in preventing HBV-related hepatitis during treatment with obinutuzumab- or rituximab-containing immunochemotherapy. Antiviral prophylaxis was effective in preventing HBV reactivation and may be an appropriate option for pts with resolved HBV infection and multiple risk factors.
Kusumoto: Chugai: Honoraria, Other: GALLIUM and GOYA are sponsored by F. Hoffmann-La Roche Ltd. Third-party medical writing support, under the direction of Shigeru Kusumoto, was provided by Cheryl Wright of Gardiner-Caldwell Communications, and was funded by F. Hoffmann-La Roche Ltd, Research Funding. Arcaini: Celgene, Roche, Sandoz: Consultancy; Gilead: Research Funding; Pfizer, Celgene, Bayer, Roche: Membership on an entity's Board of Directors or advisory committees. Kim: J&J: Research Funding; Takeda: Research Funding; Celltrion, Inc: Consultancy, Honoraria; Novartis: Research Funding; Roche: Research Funding; Donga: Research Funding; Mundipharma: Research Funding; Kyowa-Kirin: Research Funding. Peters: Genentech, Merck: Honoraria; Roche: Honoraria. Tanaka: BMS: Honoraria, Research Funding; Chugai: Honoraria, Research Funding; GSK: Honoraria, Research Funding. Zelenetz: Celgene: Consultancy; Amgen: Consultancy. Kuriki: Chugai: Employment. Fingerle-Rowson: F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Nielsen: F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Ueda: Chugai: Employment. Piper-Lepoutre: Roche: Employment. Sellam: Roche: Employment. Tobinai: Daiichi Sankyo Co., Ltd: Consultancy, Honoraria; AbbVie: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; HUYA Bioscience: Honoraria; Chugai: Honoraria, Research Funding; Eisai: Honoraria, Research Funding; GlaxoSmithKline: Research Funding; Janssen: Honoraria, Research Funding; Kyowa Hakko Kirin: Honoraria, Research Funding; Mundipharma: Honoraria, Research Funding; Ono Pharmaceutical: Honoraria, Research Funding; Servier: Research Funding; Takeda: Honoraria, Research Funding; Zenyaku Kogyo: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal